
Permanganate Anion, Chemical Structure. 3D Rendering. Atoms are Represented As Spheres with Conventional Color Coding: Manganese Stock Illustration - Illustration of oxygen, model: 188435872

MnO4 and PO4 tetrahedra in Mn3(PO4)4‚2(N4C6H21)‚ 6(H2O): MnO4 open,... | Download Scientific Diagram
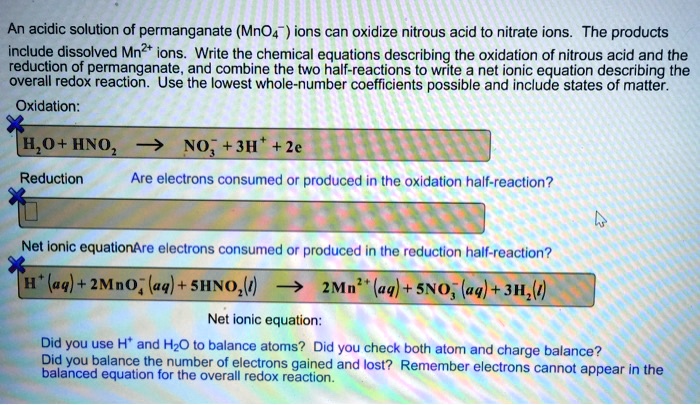
SOLVED:An acidic solution of permanganate (MnO4" ions can oxidize nitrous acid t0 nitrate ions_ The products include dissolved Mn?+ ions Write the chemical equations describing the oxidation of nitrous acid and the

How hydrogen‐bonded MnO4‐ can influence oxidation of olefins in both gas phase and solution? - Javan - 2012 - Journal of Physical Organic Chemistry - Wiley Online Library





.PNG)



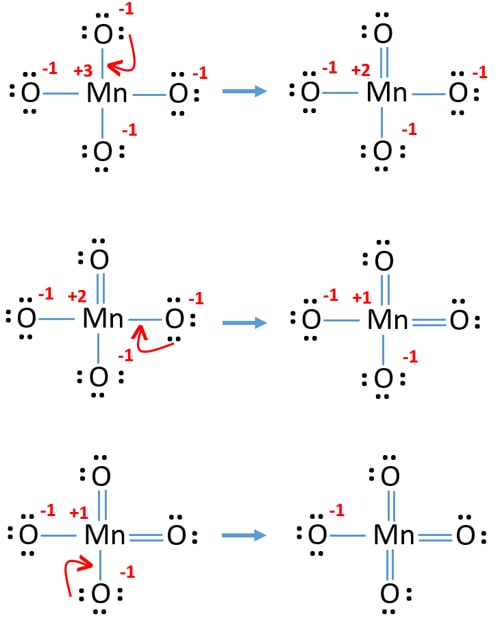






.PNG)