PDF) Determination of the Diffusion Coefficient of the Green and Violet Isomers of Cr2(SO4)3 in Aqueous Solution
![The equivalent weight of Cr2(SO4)3 [mot wt. = M] in the following reaction is Cr2(SO4)3 + H2O2 + NaOH → Na2CrO4 + Na2SO4 + H2O The equivalent weight of Cr2(SO4)3 [mot wt. = M] in the following reaction is Cr2(SO4)3 + H2O2 + NaOH → Na2CrO4 + Na2SO4 + H2O](https://d2rrqu68q7r435.cloudfront.net/images/5468271/dd6d8cae-528b-487c-a895-91facf1b047a.jpg)
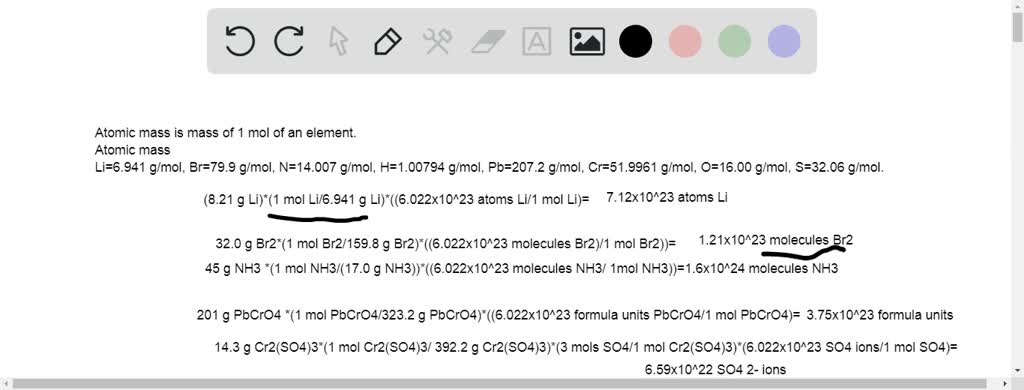
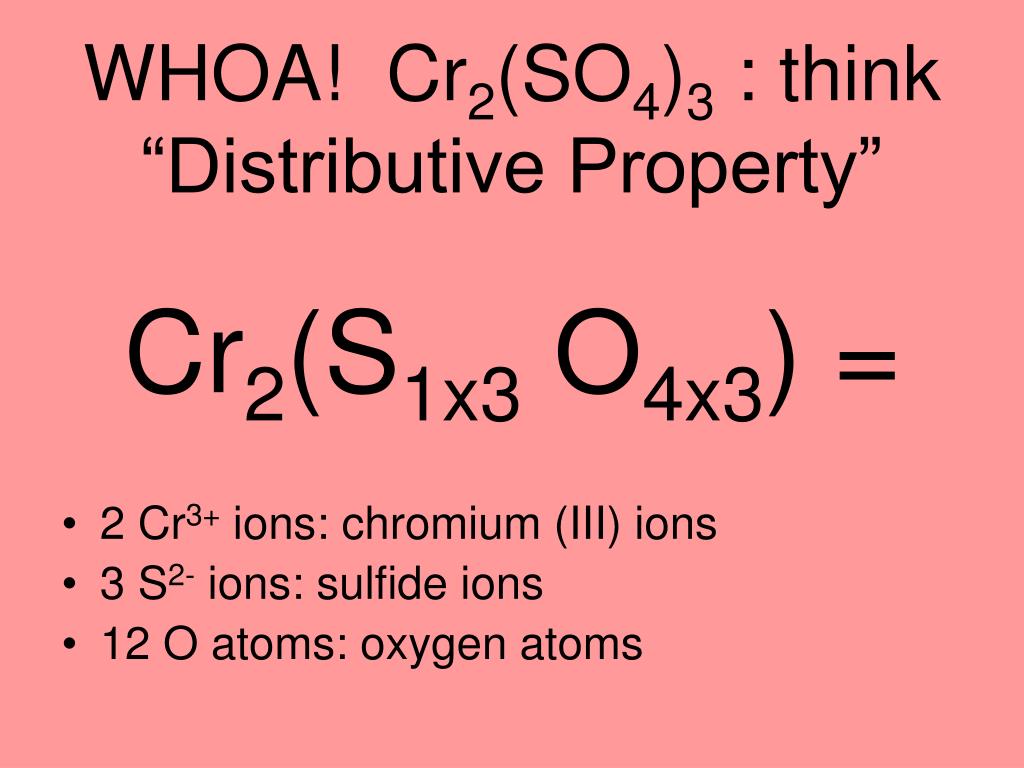
The equivalent weight of Cr2(SO4)3 [mot wt. = M] in the following reaction is Cr2(SO4)3 + H2O2 + NaOH → Na2CrO4 + Na2SO4 + H2O
![The equivalent weight of Cr2(SO4)3 [mot wt. = M] in the following reaction is Cr2(SO4)3 + H2O2 + NaOH → Na2CrO4 + Na2SO4 + H2O The equivalent weight of Cr2(SO4)3 [mot wt. = M] in the following reaction is Cr2(SO4)3 + H2O2 + NaOH → Na2CrO4 + Na2SO4 + H2O](https://haygot.s3.amazonaws.com/questions/1305019_698125_ans_e9f07e9b3a3e45088aea1d3aa5aa36ea.jpg)
The equivalent weight of Cr2(SO4)3 [mot wt. = M] in the following reaction is Cr2(SO4)3 + H2O2 + NaOH → Na2CrO4 + Na2SO4 + H2O

PDF) Aqueous Solutions of Cr(III) Sulfate: Modeling of Equilibrium Composition and Physicochemical Properties
Balance the following equations by oxidation number method 1. K2Cr2O7 + KI + H2SO2 → K2SO4 + Cr2(SO4)3 + I2 + H2O - Sarthaks eConnect | Largest Online Education Community
![The equivalent weight of Cr2(SO4)3 [mot wt. = M] in the following reaction is Cr2(SO4)3 + H2O2 + NaOH → Na2CrO4 + Na2SO4 + H2O The equivalent weight of Cr2(SO4)3 [mot wt. = M] in the following reaction is Cr2(SO4)3 + H2O2 + NaOH → Na2CrO4 + Na2SO4 + H2O](https://d2rrqu68q7r435.cloudfront.net/images/9163809/eab34822-4e6e-4ea5-9099-322d6f5d0ae5.jpg)
The equivalent weight of Cr2(SO4)3 [mot wt. = M] in the following reaction is Cr2(SO4)3 + H2O2 + NaOH → Na2CrO4 + Na2SO4 + H2O

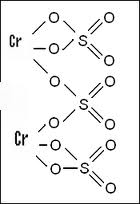
Balance the following equation by oxidation number method. PbCrO4 + H2SO4 + FeSO4→ Fe2(SO4)3 + PbSO4 + Cr2(SO4)3 + H2O